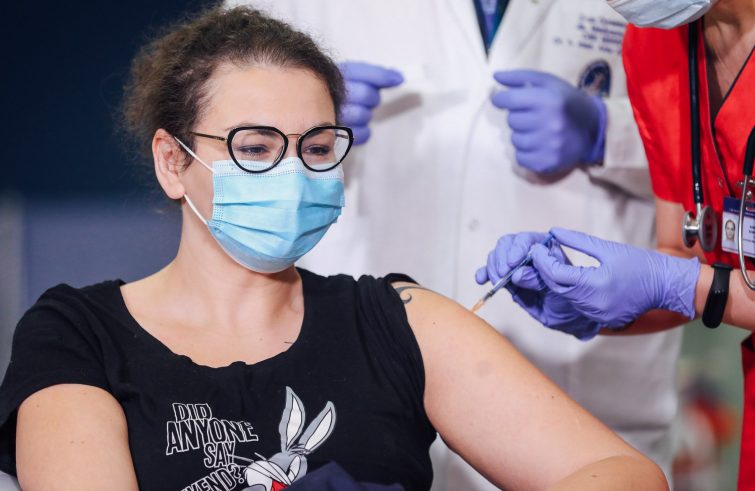
“We approved three vaccines and the distribution of doses is gradually speeding up. Member States now have to ensure that the pace of vaccinations follows suit”. This is according to a spokesperson for the European Commission who, in an interview with SIR news agency, commented on the vaccine roll-out in Europe, following the approval of the AstraZeneca vaccine, the third one to be given the green light by the European Medicines Agency (EMA). “Contracts have been signed, so States can order needles, syringes and other essential equipment”, the EU spokesperson said. At present, according to Brussels, the pace of the vaccination campaign varies from Member State to Member State due to national tools and equipment. “This is the first time in history that we have all undertaken a mass vaccination campaign of this size and complexity, and there are certainly a number of challenges along the way”, the Commission spokesperson said. “It is now up to Member States to effectively vaccinate people as quickly as possible and to ensure that the pace of vaccinations matches that of deliveries, making sure that all available doses are used”.
The Commission presented a Covid-19 vaccines strategy in mid-October, and “we have worked  with EU Member States to ensure that everyone is ready”, the representative explained. In addition, “we have launched a joint procurement procedure for the purchase of the necessary Covid-19 vaccination equipment”. According to surveys conducted in Member States before the holidays, “a majority of Member States reported that they have enough personnel to administer the vaccines”, the spokesperson continued. As for travel restrictions, the Commission does not rule out that “proportionate travel measures may also be taken in areas with a high transmission rate”.
with EU Member States to ensure that everyone is ready”, the representative explained. In addition, “we have launched a joint procurement procedure for the purchase of the necessary Covid-19 vaccination equipment”. According to surveys conducted in Member States before the holidays, “a majority of Member States reported that they have enough personnel to administer the vaccines”, the spokesperson continued. As for travel restrictions, the Commission does not rule out that “proportionate travel measures may also be taken in areas with a high transmission rate”.
